Describe Electronegativity in Your Own Words
Fluorine the most electronegative. Describe in your own words the.

Electronegativity Examples Trends Video Lesson Transcript Study Com
The Pauling scale is the most commonly used.

. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Suppose A bond is formed as a result of chemical reaction between H. Noun the tendency or a measure of the ability of an atom or molecule to attract electrons and thus form bonds.
Chemistry the tendency of an atom or radical to attract electrons in the formation of an ionic bond. This number is closely linked to. What are the four most electronegative elements and what does electronegativity mean.
Electronegativity is the tendency of an atomelement to attract a shared pair of electrons towards itself. As mentioned the electronegativity trend refers to the way electronegativity values trend across the periodic table of the elements. Examples of electronegativity in a sentence how to use it.
100 1 rating Transcribed image text. It is a dimensionless property because it is only a. Electronegativity - ˌneg- ə- ˈtiv- ət- ē.
Electronegativity is the property of an atom which increases with its tendency to attract the electrons of a bond. How to pronounce electronegative audio noun plural electronegativities. The elements range in value from 07 caesium and francium the least electronegative to 40 fluorine the most electronegative.
The protons of an atom will always have an attraction for electrons. Fluorine the most electronegative. Chapter 1 starts off with a comprehensive summary of basic chemical principles.
Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. An atoms electronegativity is affected by both its. If two bonded atoms have the same electronegativity values as.
Describe in your own words Isaac Newtons Law of Universal Gravitation. Transcribed image text. Electronegativity is a chemical property that measures the tendency of an atom to attract electrons towards itself.
The ability of an atom to attract electrons in a chemical bond. Electronegativity is affected by the atomic number and the. The tendency of an atom in a molecule to attract the shared pair of electrons towards itself is known as electronegativity.
We review their content and use your feedback to keep the quality high. Words nearby electronegativity electron camera electron capture electron carrier electron diffraction electronegative electronegativity electroneurography electroneuromyography. Electronegativity is a way to measure how much an atom attracts electrons in a chemical bond.
From WordNet 30 Copyright 2006 by Princeton University. When moving from left to right across. Show More Sentences Electronegativity refers to the ability of an atom in a compound to.
Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Each atom contributes an equal number of electrons towards the bond formation.
The electronegativity of an atom or element is the strength of attraction for more electrons by that element. The Pauling scale is the most commonly used. Electronegativities Describe the bond formed between two atoms with similar high.
Movement of electrons from one element to another. Electronegativity is a property that describes the tendency of an atom to attract electrons or electron density toward itself. It is an attraction that exists between all objects everywhere in the universe.
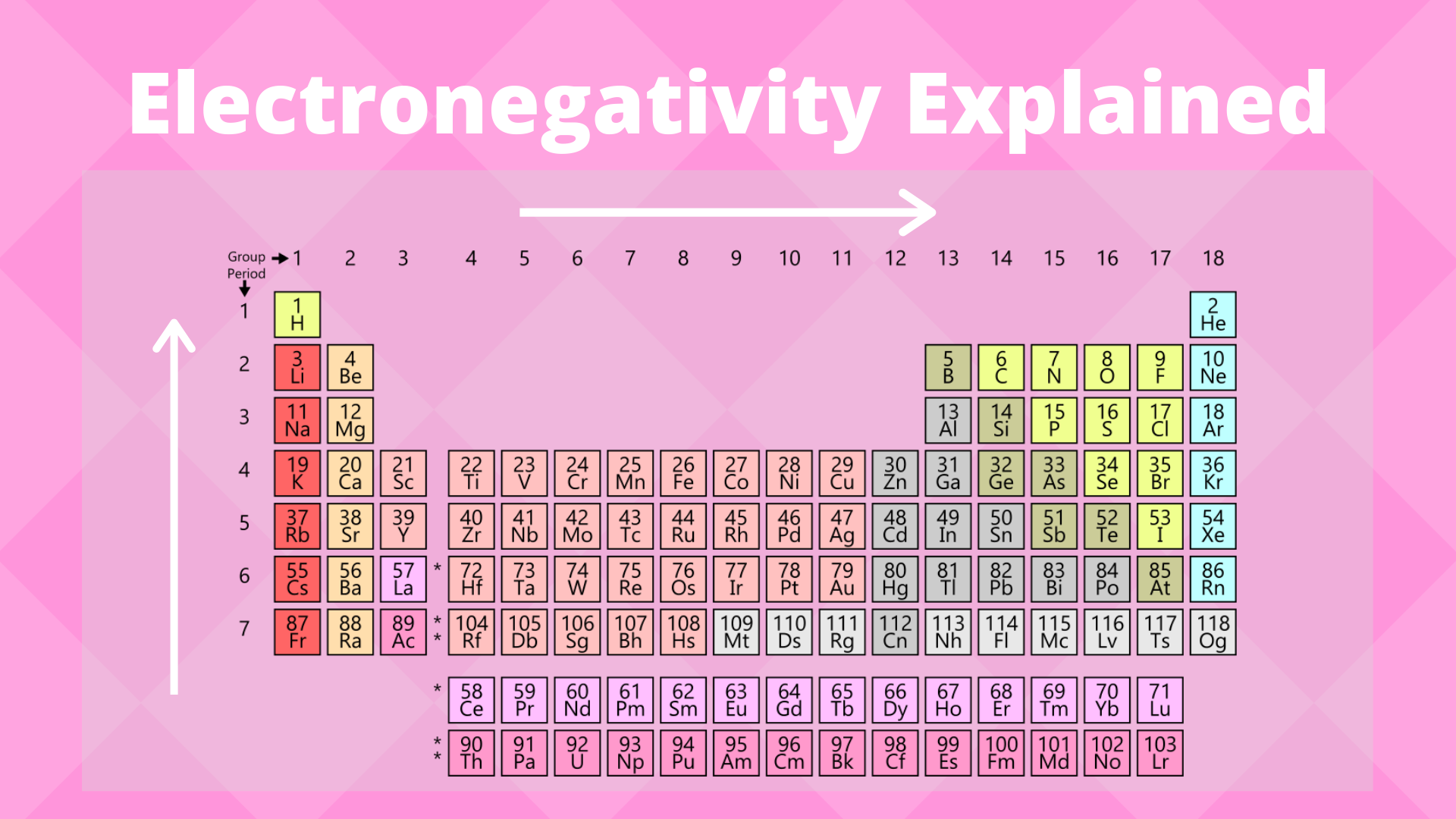
Electronegativity Trend Science Trends

What Is Electronegativity Trends Chart Periodic Table Chemtalk
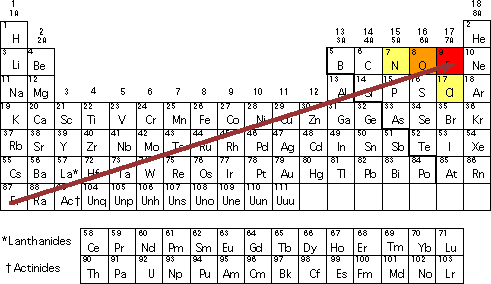
No comments for "Describe Electronegativity in Your Own Words"
Post a Comment